Over 1.1 million Cubans have received at least one dose of vaccine candidates
especiales
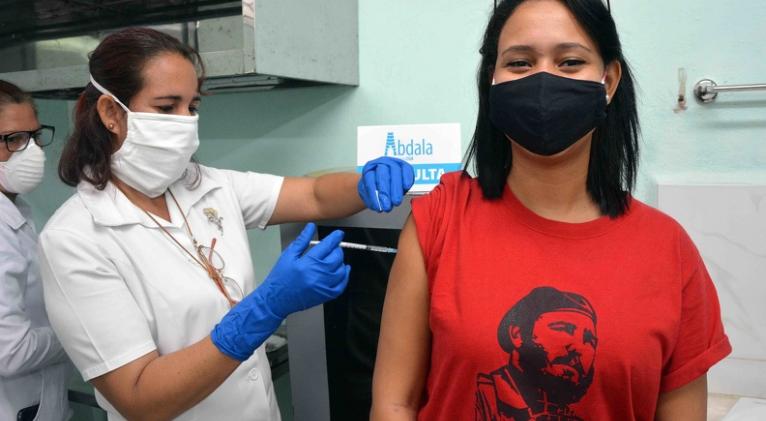
Over 1.1 million doses of the vaccine candidates Soberana 02 and CIGB-66 (Abdala) were applied in Cuba, to date, as part of clinical trials, intervention study and health intervention in at-risk groups and territories.
Iliana Morales, director of Science and Technological Innovation of the Ministry of Public Health (Minsap), said that 172,233 of them included the trials developed in several municipalities of Havana and Granma, Santiago de Cuba and Guantánamo, in the southeastern part of the country.
The doctor added that 330,726 doses are accounted for in the intervention study on health personnel and the biopharmaceutical sector, extended to several localities of the island, while 612,376 correspond to the health intervention in groups and territories at risk, announced in recent days by José Ángel Portal, head of the Minsap.
He said that in more than 60 percent of the nation's clinical sites the inoculation of at least the first dose of the two most advanced anti-COVID-19 vaccine candidates of the five developed by the Cuban scientific community was concluded.
Morales asserted the maintenance of the periods set by the highest health authority in the country for the interventions, but warned that no immunization site will open without the availability of the required material and human resources because the process will not stop once it has begun.
In this sense, she acknowledged the work of scientists, the Cuban biopharmaceutical industry, the health system, transporters and all the personnel who in one way or another contribute to the success of vaccination in its different variants.
Incidentally, she commented on the existence of similarities and differences between the types of studies and interventions that aim, at the end of the day, at protection with their own vaccines against disease caused by the SARS-Cov-2 virus.
She pointed out, as points in common, compliance with approved ethical standards - principle of voluntariness and informed consent of the participants - and controlled by the Research Ethics Committees, the fact of having methodological procedures for their organization and with a schedule and assurance plan.
The protocols, inclusion and exclusion criteria, and the complexity of the data and its statistical processing differentiate them, although it was agreed to maintain the last aspect for all of them, given the traceability and reliability that it grants them, she pointed out.
The Minsap official emphasized Cuba's adherence to the requirements established by the national and international regulatory authorities for research of this magnitude, as it began with the clinical trials in their three phases, in order to demonstrate safety, immunogenicity and efficacy.
These were followed by intervention and health intervention studies, after the first two indicators had been proven.
Iliana Morales underlined the challenge that the latter constitutes for family doctors and nurses, who have become the protagonists of one of the greatest feats of the Cuban health system: the fight against the new coronavirus.
She added that the anti-COVID-19 vaccination does not imply the suspension of the rest of the programs, such as the anti-vectorial campaign and mother and child care, of vital importance in the preservation of the citizens' health.
Finally, she insisted that the health intervention is expected to affect the population groups and territories with the highest epidemiological risk in Havana, Matanzas, Pinar del Río and Santiago de Cuba, in principle, until the whole island is covered.













Add new comment