Cuban vaccine Quimi-Vio
especiales
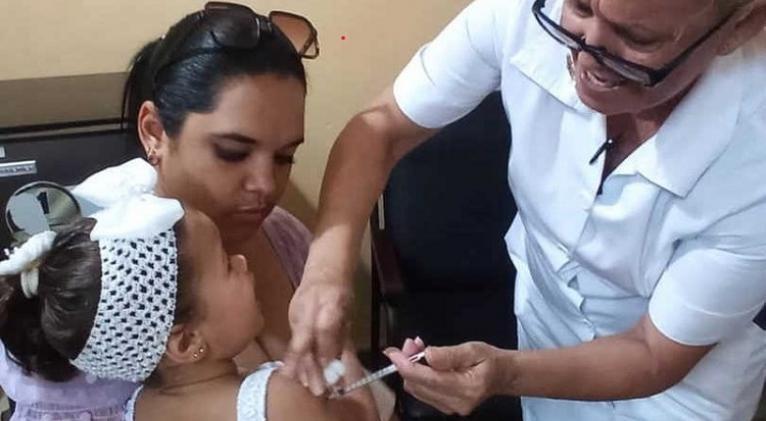
The announcement resonated in the room. A great sigh of relief, satisfaction, and prolonged applause filled the following minutes. It was the first recognition of more than a decade of intense and exhausting research, advances, setbacks, highlights, shadows, clinical trials, hopes, doubts, certainties…
“It was one of the most exciting days of my career. The impact of the news marked one of the most transcendental moments in the professional and personal lives of those of us who participated in the entire process.”
This is how Dr. Rinaldo Puga Gómez describes the moment when the multivalent immunogen Quimi-Vio against pneumococcus received Sanitary Registration for use in children between one and five years of age.

This prestigious pediatric specialist has no shortage of arguments when it comes to defining what the approval of this injectable developed at Finlay Vaccine Institute (FVI) meant for the medical and scientific community. In an exclusive interview with CubaSí, he details the experience.
“For several generations of pediatricians, family doctors, and health authorities at the ministerial level, we have dreamed of having our own vaccine to prevent invasive pneumococcal disease and the carrier state. From now on, we have a tool that will fundamentally impact the reduction of infant morbidity and mortality in Cuba. It will also reduce the hospital burden by reducing admissions.”

The validation of Quimi-Vio went public on July 5, 2024. As the regulatory entity in the country for this category, the CECMED (Center for the Control of Medicines, Equipment, and Medical Devices) had to thoroughly evaluate all the stages of research and clinical trials required to demonstrate the product's quality, efficacy, and safety.
As leading clinical investigator in the development of the pediatric vaccine, Dr. Puga knows very well how much effort has been invested over 12 years of hard studies.
“The clinical trials began in 2012. Phase 1 was first administered to healthy, non-adolescent young adults in Havana. Since then, it has been analyzed in people of different ages: preschoolers, infants in the first and second semesters of life, and children with comorbidities or illnesses.
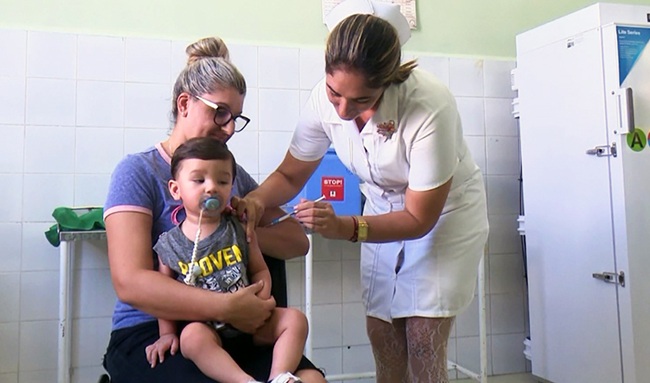
“However, the most important thing is that a multicenter study was conducted that included, first and foremost, various studies primarily at the Juan Manuel Márquez Children's Hospital; although we also included some polyclinics in the capital. Later, we repeated a second study, at Juan Manuel Márquez Center itself, with a view to stabilizing future production of the pneumococcal vaccine.
Just as the testing period in Havana was concluded and everything was ready to begin administering the vaccine candidate, the devastating COVID-19 pandemic broke out on the island.
What followed was the hiatus of almost all sectors of society and projects, including the immunogen. The priority then was to dedicate every resource and human effort to fighting the SARS-CoV-2 virus.
Attempting to do big things
Backed by a prestigious medical career, Dr. Rinaldo Puga, born in May 1962, has accumulated four decades of pediatric work combined with teaching, and more than 13 years in the field of clinical research.
“For me, the opportunity to delve into the world of so many details, requirements, rigor, and requisites applied by the FVI is priceless. In particular, the professional solidity when developing a vaccine, taking into account all the high international standards required by regulatory institutions.
“This is one of the reasons why it took us more than 10 years just for clinical trials, and more than 20 since the process began to obtain the pneumococcal vaccine. At the laboratory level, Professor Vicente Vérez led the initial work from 2002-2003.”
“In my case, I participated in the entire clinical research.” on the pediatric side (meetings, desk job and field work with children). We owe these children their collaboration, but above all, their parents, who trusted our team and agreed to allow their children to participate in the trials.”
Regarding the operating protocol, Dr. Puga explains that after administering a dose of the vaccine, clinical follow-up is established for the infant. “This is the procedure required internationally to monitor potential adverse events associated with immunization.”
Regarding his ties with the FVI, this first- and second-degree specialist in Pediatrics and a second-degree specialist in Immunology recalls that they arose from an International Symposium on pneumococcal disease held in Brazil. Later, that relationship would become stronger during the research processes for the anti-coronavirus injectables Soberana 02 and Soberana Plus.

In fact, in 2023 he received the Annual Award from the Cuban Academy of Sciences for participating as a leading investigator in the results obtained with these pediatric immunizers. Such experiences serve as a platform for him to defend his PhD in Science today.
Enemy Lurking
Invasive disease caused by the pneumococcus bacteria, or Streptococcus pneumoniae, contributes to the occurrence of otitis, sinusitis, pneumonia, upper respiratory and bloodstream infections, and meningoencephalitis, Dr. Puga points out.
“Globally, there’s respiratory syncytial virus (a common viral infection with mild symptoms similar to the common flu, although it can cause severe lung damage). Then comes a second wave of pneumococcal infections. In other words, invasive serotypes circulate in all countries; children are the most vulnerable. Cuba is at the same level as what is happening worldwide with this germ.”
Hence the relevance of the Caribbean nation having Quimi-Vio, whose name pays tribute to the prestigious (late) scientist Violeta Fernández, one of the leading researchers in the project's preclinical development.
According to the expert, one of the advantages of a vaccine of this type is that it meets the non-inferiority criteria. "This concept means that our product is not inferior in efficiency, effectiveness, and safety to those available in other countries.
"Similarly, the fact that it’s a conjugated immunogen allows its use in young adults, minors, and adults in their third and fourth ages. Although it’s true that applying it to children would lead to a herd immunity effect, which guarantees a reduction in the infectious burden in other age groups.
“Likewise, in social and economic terms, it represents an extreme human value that cannot be measured, as it’s a disease that tends to cause death or leave a large number of after-effects. All of this impacts parents' work attendance, the loss of schooling, and lifelong health complications for the individual…”
As an advocate of preventive medicine, the pediatrician warns and advises that prevention is always essential. “We recommend, above all, personal protection, handwashing, not sending a sick child to daycare or school, and avoiding crowds, among other measures.”

However, the work and scientific talent of these Cuban researchers persist in their mission to guarantee the care of children's lives. And with them, Dr. Rinaldo Puga is on the front lines.
“Our goal is to expand the vaccine from seven serotypes to eleven. This would increase coverage to prevent the impact of pneumococcal disease in children. For now, it will only be administered to children two years of age or older.”
Translated by Amilkal Labañino / CubaSí Translation Staff













Add new comment