Official: Pasteurcovac Vaccine Efficient against African Variant of Coronavirus
especiales
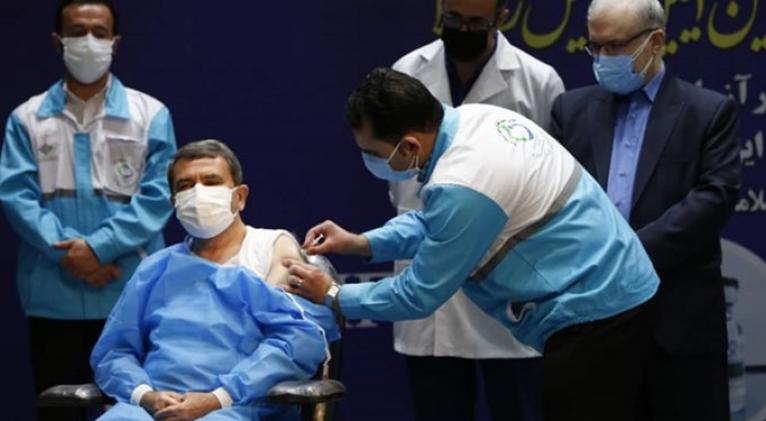
“The results of studies conducted in Cuba showed that the vaccine still retains its immunogenicity against common variants,” Mostafavi said on Saturday.
He added that in the third phase of clinical studies in Cuba, where the most common epidemic mutation is the South African variant, on a vaccine-avoiding variant, the results of studies were promising and showed effectiveness of the vaccine.
“Based on available reports, 62% efficacy of two doses of the vaccine was obtained in volunteers participating in the third phase of clinical trials of the vaccine in Cuba,” Mostafavi said.
Mostafavi said late last week that Pasteur Institute of Iran will produce and supply the health ministry with 3mln doses of Pasteurcovac vaccine within 3 months.
“3 million doses of vaccine will be delivered to the health ministry by the end of Summer, but after boosting the capacity, we hope to increase production by 2 to 3 times in early fall,” Mostafavi said last Sunday.
He added that production of the vaccine at Pasteur Institute started concurrently after the 3rd phase of its clinical trial began, noting that the license for emergency injection of Pasteurcovac vaccine will be issued soon.
Mostafavi said last month that reports suggest the Iran-Cuba joint COVID-19 vaccine has proven to be effective by over 62 percent in the third phase of the clinical test carried out in Cuba.
"The vaccine which is known as Pasteurcovac in Iran and Soberana-2 in Cuba has proved 62 percent effective based on results from the second-dose injections in the third phase of the clinical test which was done in Cuba," he added.
He said that the vaccine had undergone clinical test in Cuba with a 28-day plan of two doses and another 28-day plan of two doses and a booster (with Soberana+ vaccine candidate) on 44,000 volunteers in Cuba and 24,000 volunteers in Iran.
Mostafavi stressed that none of the existing vaccines are 100 percent effective, as some of the people receiving them are infected again and in some rare cases, severe infections and deaths are reported.
Global regulators have announced over 50 percent effectiveness as acceptable and the effectiveness of a vaccine depends on different factors, including its design and production and the types of viruses in circulation.
Given the different strains of viruses in different countries and the coronavirus' mutations in the same location over time, any vaccine might have different levels of effectiveness in different conditions.
In relevant remarks in late May, the head of Iran's Pasteur Institute, Alireza Biglari said that the Iran-Cuba jointly produced COVID vaccine is in its clinical study's final phase.
“The initial injection of the joint vaccine of the Pasteur Institute of Iran and the Finlay Institute of Cuba, which is in the final phase, has begun for 24,000 Iranians,” Biglari said on May 30, adding that the first dose of vaccine had been injected for 24,000 Iranians in seven provinces and eight cities.
He went on to say that the vaccine has successfully completed phases one and two of clinical studies in Cuba, and the third phase is being conducted simultaneously in Iran and Cuba.













Add new comment